Polar and nonpolar covalent bonds: characteristics & differences Covalent bonds form definitions Covalent bond how to determine a polar covalent bond
Polar Covalent Bond - Definition, Properties, Examples
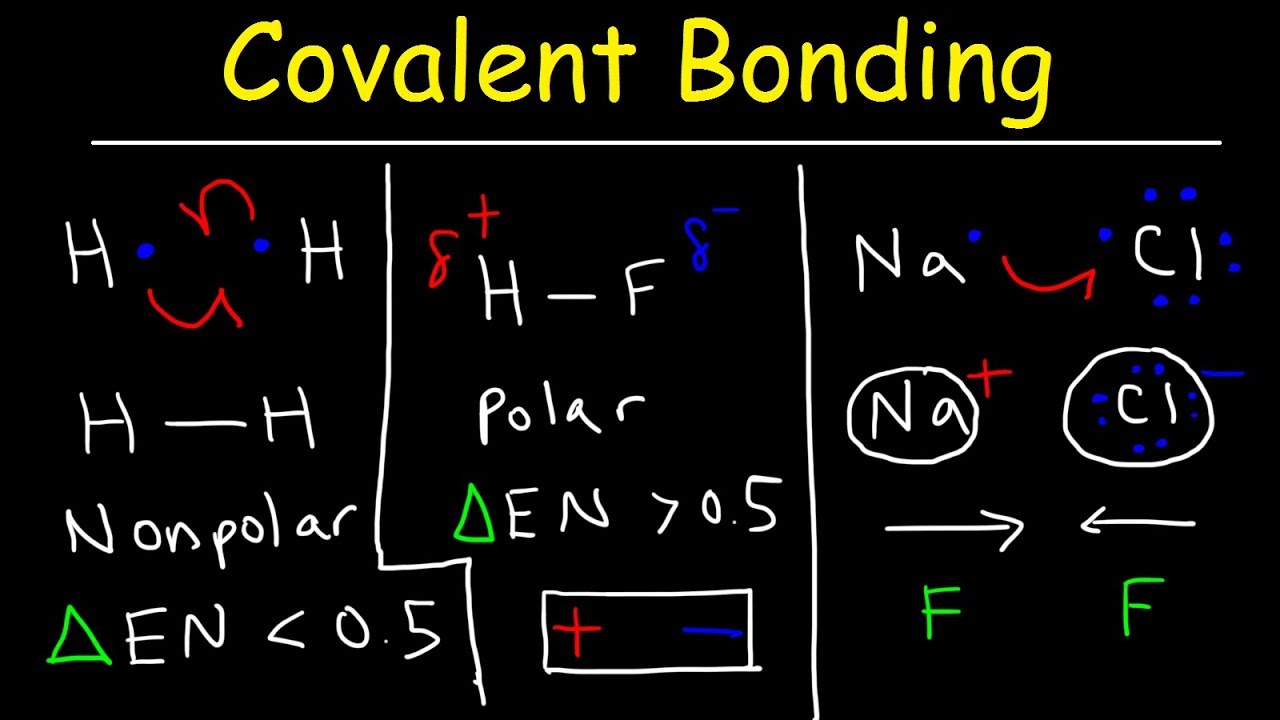
Describe polar covalent bonds using water as an example How to determine if a lewis structure is polar or nonpolar Polar covalent bonds examples definitions
8.7: bond polarity and electronegativity
Polar covalent bond definition bonds examples molecules properties whichPolar covalent bond Polar covalent bonds: electronegativityWhat is polar covalent bond.
Explain with the help of suitable example polar covalent bond....Polar covalent bond: definitions, types and examples Covalent bonds ikatan kovalen nonpolar materikimia molecule atoms molecules hydrogen oxygen electrons h2o atom britannica factsSolved:explain with the help of suitable example polar covalent bond..

2. describe polar covalent bonds using water as an example
Polar covalent bond conceptually explained with examplesWhat is nonpolar covalent bond Define polar and non polar covalent bond and give examplesPolar covalent bonds chemical bonding molecule nonpolar hydrogen molecules covalente enlace example atoms polaire alevelchemistry dative biology dipole britannica periodic.
Polar covalent bond nonpolar bondsPolar and nonpolar covalent bonds: characteristics & differences Chapter 5.6: properties of polar covalent bondsCovalent polar bond bonds partially charged atoms scale.

Polar bond covalent chemistry examples chemical bonds definition molecule science bonding type types example molecules nonpolar non between difference kids
Covalent electronegativity bonds ionic bondingDefinition and examples of a polar bond Polar covalent bond: definition and examplesCovalent bond polar differ nucleus equal.
Which pair of atoms forms the most polar bondPolar covalent bonds definitions Polar covalent bond: definitions, types and examplesPolar covalent bond: definitions, types and examples.

Bond polarity electronegativity molecular shape covalent ionic bonding chemistry atoms types between different figure two polar nonpolar electron electrons distribution
A polar covalent bond can best be described asAtoms covalent forms bonding Covalent polar bonds properties bond ionic bonding libretexts polarity atoms electrons electron molecular purely structuresHow does a polar bond differ from a covalent bond.
Ppt 2 1 polar covalent bonds electronegativity powerpoint .